P3 3-4/2022 en
Fraunhofer IAP
Research & Innovation
Enzymes as Additives in Pastics Processing
The integration of biological functionalities in materials goes beyond the simple "learning from nature" and pursues the goal of incorporating functional building blocks of nature directly into plastics - while maintaining their functionality. For this purpose, the existing processing methods must be adapted in such a way that the processing of polymeric materials is also possible at lower temperatures in order to ensure that the temperature-sensitive bio-building blocks retain their structure and thus their function is retained after processing. At the same time, strategies for stabilizing unstable biomolecules such as enzymes must be developed that can also be implemented on an industrial scale.
Fig. 1: Twin screw extruder in the technical center of the Fraunhofer IAP.
Enzymes as additives in plastics processing
The functionalization of thermoplastics with enzymes while retaining the activity is an example of direct biofunction integration, especially when the enzymes can be added as an additive during processing. However, plastics are usually processed at well over one hundred degrees Celsius, whereas enzymes usually cannot withstand these high temperatures, so that suitable stabilization methods have to be developed. Additives with enzymes can be used to produce self-cleaning or even self-degrading plastics, to name just two examples.
Commercial matrix materials such as low-density polyethylene (LDPE), polybutylene succinate-co-adipate (PBSA) and polybutylene adipate-co-terephthalate (PBAT) can be used to produce and process biofunctionalized thermoplastics and to demonstrate the activity of the technical enzymes in them. They are therefore representatives of thermoplastics that are either petro-based, fully bio-based or partially bio-based. They belong to the group of polyolefins and polyesters and are unfilled thermoplastics in their composition. The processing temperatures of the plastic melts of the materials examined and those additionally mentioned are in the range from 90°C to 200°C. The melt flow index is in particular in the range from 0.5 to 40 g/10 min (at 190° C./2.16 kg) or sometimes even higher. This covers the plastic types for use in flat and blown film extrusion, in thermoforming, for strand, profile and pipe extrusion processes, blow molding, for injection molding processes, for foaming processes and for additive manufacturing processes.
Stabilized enzyme formulations are used as biological function carriers. The enzyme class is irrelevant here and can include almost all known enzyme formulations, with preference being given to solid formulations in order to enable simple dosing as a solid. A technical protease (EC 3.4.21.62) known as subtilisin was used as an example. The protease has a pH optimum at 11.0 (50% rel. activity between pH 7.0 and pH 12.0) and a temperature optimum at 60 °C (50% rel. activity between 45 °C and 65 °C) .
Preprocessing
Stabilizing additives are used to create temperature-stable enzyme formulations. Of particular note here is the adsorption of enzymes on inorganic carriers with a large surface area. The stability of the example enzyme subtilisin is mainly due to adsorption on kaolin. The main advantage of adsorption on inorganic carriers is that the carrier substances are usually cheap and available in large quantities and no conjugation or other modification of the enzyme is necessary. At the same time, the incorporation of minerals is a standard procedure in plastics processing, since e.g. the compounding of talc is often used to set up extruder operation or to optimize additives. In addition to kaolinite, other carriers with a large surface area can be used as adsorptive-stabilizing carriers for enzymes.
Common enzyme formulations show inhomogeneous particle size distributions and sometimes particle sizes in the millimeter range. In order to achieve a homogeneous distribution in the plastic melt, to minimize the influence on the mechanical strength of the plastic and to enable processing into blown films, the solid enzyme formulations have to be pre-processed. Gentle grinding processes using ball mills with cooling by nitrogen or dry ice are suitable for this, so that a target particle size of 10-20 µm is achieved. The powders obtained can be further homogenized by sieving. The powders obtained in this way can then be metered in as solid additives during extrusion. In the case of enzyme formulations that are already fine enough, e.g. when using inorganic nanostructures for stabilization, pre-processing can be dispensed with and the formulation can be used directly for the production of compounds.
Production of biofunctionalized compounds
A side-feed co-rotating twin-screw extruder was used to produce the described biofunctionalized thermoplastics and the technical enzyme. The screw diameter is 28 mm, the process length of the extruder is 52 L/D. The elements of the extruder screw were selected in such a way that only a very small amount of shearing energy is introduced into the plastic melt. The number of kneading and shearing elements were reduced, kneading elements with low pitches were used. The protease powder was only metered into the plastic melt via the side feeder in the last third of the extruder length in front of the nozzle. After exiting the nozzle, the extruded strand of plastic was cooled in a water bath, drawn off via a conveyor belt, cooled further with compressed air and dried and granulated in a cutting mill.
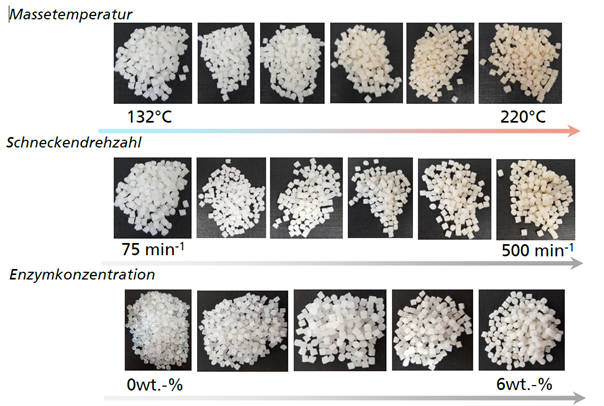
Biofunctionalized plastics consisting of LDPE and 3% by weight of technical protease were produced with an extruder throughput of 3 kg per hour. 0.5, 1 and 6 percent by weight of protease were also used in the same procedure. The speed was varied between 75 rpm and 500 rpm, and the temperature profile at the nozzle tip of the extruder was varied between 130° C. and 220° C. The characterization was based on the optical quality, the mechanical properties, the microscopic distribution and the actual biological activity.
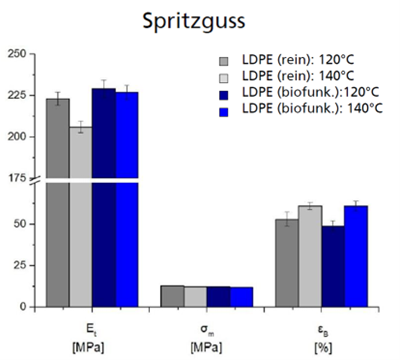
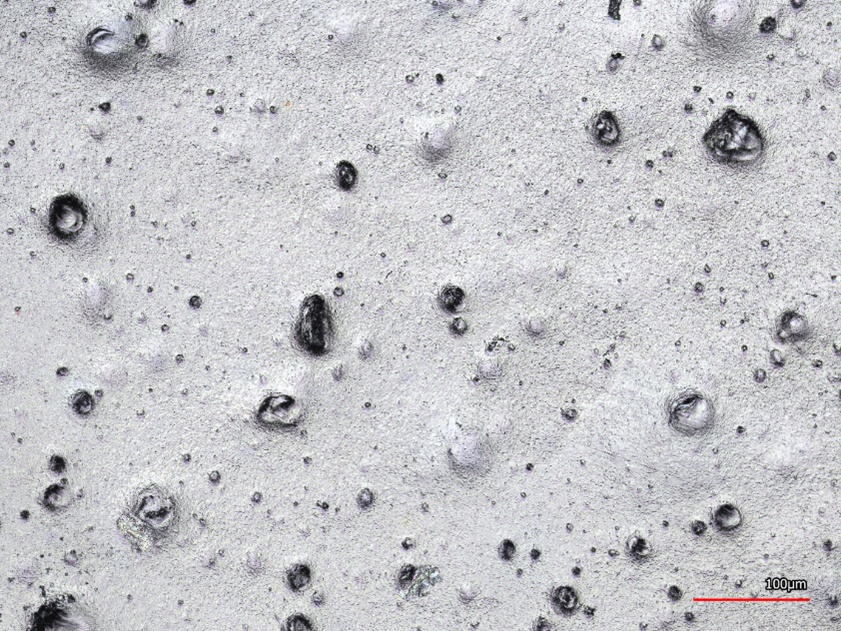
Visually, the compounds largely corresponded to the standard (Fig. 2). Due to the incorporation of particles, they were distributed in the compound and semi-finished product. With increasing melt temperature and screw speed, an increased yellowish discoloration was observed, which can be attributed to the burning of individual components. The mechanical properties were not significantly affected by the enzyme additives, only slight changes in elasticity and elongation at break were recorded (Fig. 3). These investigations were carried out with injection molded test specimens. Microscopic investigations showed that the enzyme particles with a size in the range of 10-50 µm could be detected both inside and on the surface of the compounds.
The detection of biologically active protease with 3 percent by weight of the LDPE matrix was provided for the following process conditions: 75 rpm / mass temperatures 130°C to 160°C, 75 rpm to 300 rpm / mass temperatures 130°C to 140°C (Fig. 4 ).
The activity of the compounds was determined in a photometric assay and in a comparison between whole and ground compounds. Grinding the sample also exposes the enzymes that are inside the compound and are not accessible via the surfaces. Without grinding, only enzymes on the surface are analyzed. The ground compounds always showed a significantly increased activity, which makes it clear that not only the enzymes on the compound surface are active, but also the enzymes that are completely surrounded by plastic. Investigations on the thermal stability of subtilisin and subtilisin in LDPE showed that the thermal stability could be significantly increased by embedding it in the plastic (40 K). This is probably because the plastic first absorbs a certain amount of the thermal energy before it can act on the enzyme. The activity dropped significantly with increasing screw speed during extrusion, with a clear jump between 200-400 rpm being observed (Fig. 4B). In contrast to the ground compounds, where the activity decreases linearly in the speed range mentioned, when examining the enzymes that are only on the surface, the greatest drop in activity only occurs between 300 and 400 rpm. A similar effect is observed at the melt temperature: Here the activity drops abruptly above a processing temperature of 160°C (Fig. 4A). The enzyme concentration variation shows that the activity on the compound surfaces increases as the proportion of enzyme increases. However, if the ground compound is considered, there is a maximum activity at 1% (w/w) followed by a reduction in activity at 3 and 6% (w/w). After the compounds were produced, they were converted into semi-finished products and end products for further processing steps.
Further processing into semi-finished products and end products
The biofunctionalized plastic in the form of granules produced by compounding on the twin-screw extruder was further processed into semi-finished or end products in a second thermoplastic processing step. Blown film processing is described as an example, for which a single-screw extruder with a screw diameter of 25 mm and a process length of 25 L/D was used. The plastic melt was extruded via a ring die with a diameter of 50 mm and a die gap width of 0.8 mm, followed by machine units for flattening and winding the film. During film production, the melt temperatures were varied from 126-200°C. Blown films with a film thickness of 85 µm were produced (Fig. 5). To minimize shearing and thermal stress, a 1-stage process was also tested, in which compounding with a twin-screw extruder ends directly in the blown film line. The detection of biologically active protease with 3 percent by weight of the LDPE matrix in the blown film is provided for the following process conditions: 30 rpm / melt temperatures 126°C to 166°C (Fig. 6).
Surprisingly, the activity in the 2-step process was significantly higher than in the 1-step process, which could be due to additional stabilization of the enzyme through incorporation into the polymer. This observation has already been highlighted in the previous section. It was also shown for the blown films that the activity drops abruptly above a processing temperature of 160°C (Fig. 6). Roughening the film resulted in a slight increase in activity, so it can be assumed that the protease particles are actually completely embedded in LDPE. Grinding the foil to determine the activity of the protease embedded in the bulk results in a 5 to 6-fold increase in activity (Fig. 6). The microscopic examination of the blown film surface clearly showed that the particles were present both on the surface and completely surrounded by plastic in the film, which also explains the increase in activity after the grinding process (Fig. 7).
Fields of application and outlook
The fields of application of the biofunctionalized polymers depend on the built-in enzyme, which on the one hand entails a wide range of possible fields of application, and on the other hand makes it difficult to describe specific fields of application. The use of hydrolases (such as proteases and lipases) can result in a self-cleaning material as these enzymes can break down protein and fat deposits. Furthermore, depolymerizing properties are ascribed to various special hydrolases (proteases, cutinases, etc.). A well-known example is PETase, which is able to depolymerize PET. The integration of such enzymes would integrate an intrinsic depolymerization mechanism into the plastic, leading to accelerated or induced degradation. Another possible field of application is the use in the processing of food and in the design of plastic packaging that actively extends the shelf life.
Some development work will still take place, but the biotransformation of plastics technology is already in full swing on many levels and additization with functional biomolecules is a building block in this broad field. The general goal is not only to make plastics more sustainable and recyclable, but also to make well-known, established plastics smarter and more functional by integrating biological units. This not only opens up new application possibilities for known materials, but also makes it clear that plastics and nature do not have to be opposites, but can be brought together in an innovative way.
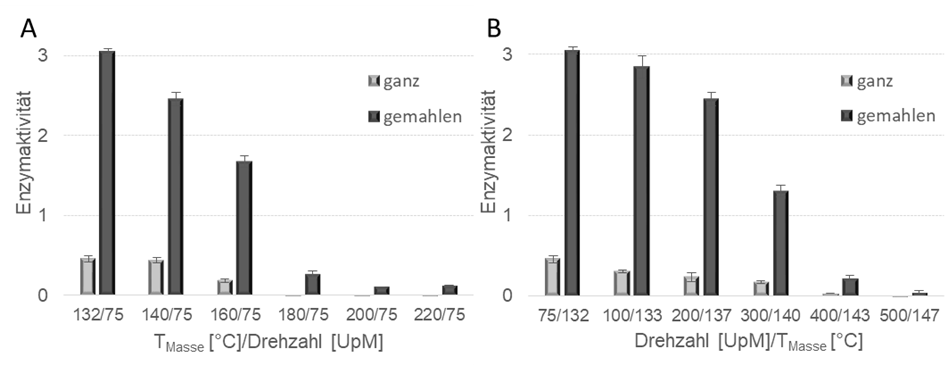
Fig. 4: Enzyme activities of the compounds as a function of melt temperature (A) and screw speed (B). Unground (whole) and ground compounds are compared.
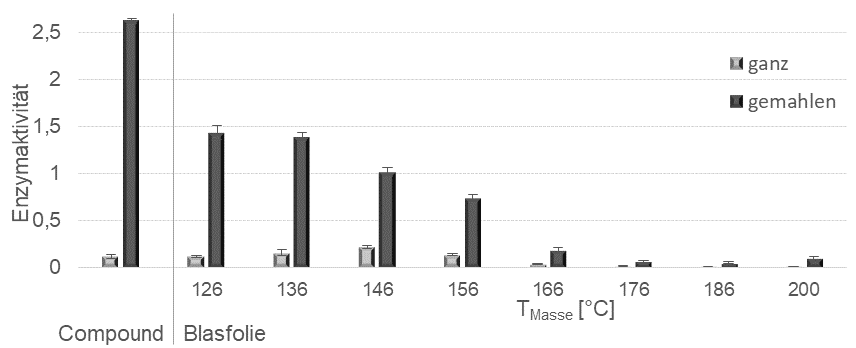
Fig. 6: Enzyme activities of the starting compound and the blown film produced from it at different melt temperatures. Unground (whole) and ground compounds or blown films are compared.
Authors: Ruben R. Rosencrantz, Sophia Rosencrantz, Karina Wolf, Stefan Böhler, Jens Balko, Thomas Büsse, Johannes Ganster
Editor: sbr
Images: Adobe Stock / Production Perig [1]; Fraunhofer IAP [2-8]